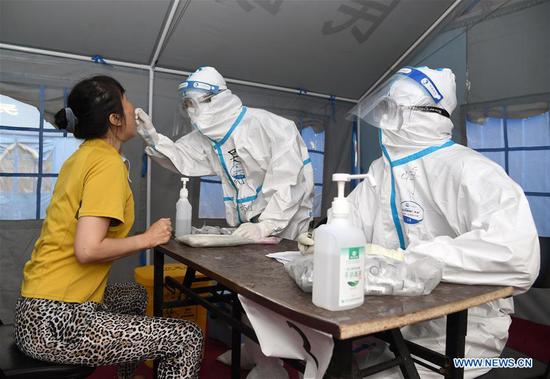
Chinese authorities have approved a new COVID-19 vaccine candidate for human trials, the Institute of Microbiology under the Chinese Academy of Sciences, said in a statement.
The recombinant protein vaccine was issued with a clinical research permit from the National Medical Products Administration on June 19, according to the institute, which is the vaccine's main developer.
Researchers will start phase-1 clinical trials in hospitals in Chongqing and Beijing, to determine whether the vaccine is safe for use on humans, its co-developer Anhui Zhifei Longcom Biopharmaceutical Co., Ltd. confirmed.
It represents the third type of COVID-19 vaccine developed by Chinese researchers to enter clinical trials. One adenovirus vaccine and four inactivated vaccines have previously been approved for human tests in China, with some having just cleared phase-2 trials.